Publiko pinag-iingat vs ‘gamot’ kontra Covid
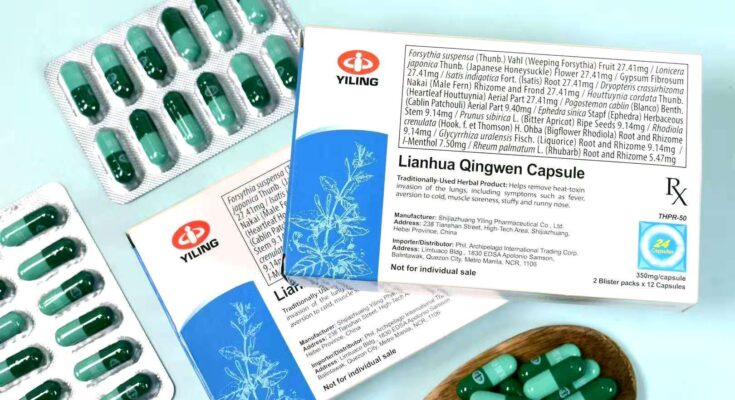
BINALAAN ng Food and Drug Administration ang publiko sa pagbebenta ng hindi rehistradong Lianhua Qingwen Jiaonang na mayroong Chinese characters.
“As per continuing post-marketing surveillance, there is still incessant sale and distribution even through social media platforms such as Facebook, of Lianhua Qingwen Jiaonang with Chinese characters, which were verified as unregistered by the FDA,” ayon sa kalatas ng FDA.
Idinagdag ng ahensya na tanging mga gamot na may English text at detalye lamang ang inaprubahang ibenta sa bansa.
“The agency cannot guarantee the quality and safety of the product with the Chinese text due to these have not undergone evaluation by the FDA and came from unlicensed sources or establishment. The consumption of such violative product may pose potential danger or injury if administered,” dagdag nito.
“All concerned establishments are warned not to distribute, advertise, or sell the said violative medical device products until the Product Notification Certificates are issued, otherwise, regulatory actions and sanctions shall be strictly pursued,” hirit pa ng FDA.
Matatandaan na isang dalubhasa ang nagbuking na hindi gamot sa Covid-19 ang Lianhua Qingwen Jiaonang.